Perma Pure’s Nafion™ polymer is often referred the wonder material for its unique ability to transfer moisture from one side of the membrane to the other. This transfer occurs as a first order kinetic reaction making it highly selective.
What is Nafion™ Polymer
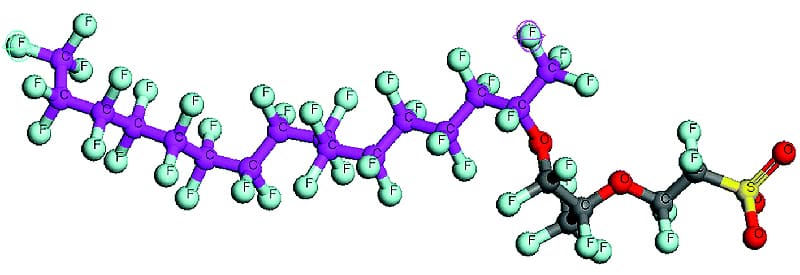
The Nafion™ polymer was invented by Walther Groth in late 1960s. Nafion™ is a copolymer of perfluoro-3,6-dioxa-4-methyl-7octene-sulfonic acid and tetrafluoroethylene (Teflon™). In simpler terms Nafion™ is a Teflon™ backbone with occasional side chains of another fluorocarbon. The side chain terminates in a sulfonic acid (-SO3H).
Apart from the sulfonic acid group, the Nafion™ polymer is a fluorocarbon. Like most fluoropolymers, it is extremely resistant to chemical attack (corrosion resistant). The sulfonic acid group is immobilized within the bulk fluorocarbon matrix and cannot be removed, but unlike the fluorocarbon matrix the sulfonic acid groups do participate in chemical reactions. The presence of the sulfonic acid adds three important properties to Nafion:
- Nafion™ polymer functions as an acid catalyst due to the strongly acid properties of the sulfonic acid group.
- Nafion™ polymer functions as an ion exchange resin when exposed to solutions.
- Nafion™ polymer very readily absorbs water, from the vapor phase or from the liquid phase. Each sulfonic acid group will absorb up to 13 molecules of water. The sulfonic acid groups form ionic channels through the bulk hydrophobic polymer, and water is very readily transported through these channels. Nafion™ polymer functions like a very selective, semi-permeable membrane to water vapor.
The physical properties of Nafion™ polymer are similar to those of other fluoropolymers. It is translucent plastic. When used as an ion exchange membrane, it operates at temperatures up to 190°C. When used as a dryer, it can operate up to 150°C. The burst pressure of Nafion™ tubing is generally greater than 200 psig (over 13 bar) but it varies with the diameter and wall thickness. An unusual property of Nafion™ is its propensity to change in physical size. As Nafion™ absorbs water, it swells by up to 22%. When exposed to alcohols it increases in size by up to 88%.
How Nafion™ Polymer Works
The Nafion™ polymer transfers water molecules from one side of the membrane to the other by a first order kinetic reaction. This is unlike a porous membrane that depends on the physical size of molecules. Nafion™ Polymer selectivity is based on chemical reactivity, not the size of the molecule – therefore, not traditional permeation. The Nafion™ polymer selectively transfers water vapors, leaving most analytes in the gas stream untouched. The driving force for the Nafion™ polymer to transfer water molecules is the difference in humidity levels on either side of its membrane. The Nafion™ polymer tries to attain equilibrium of the partial pressure of water vapor on either side of its membrane.
Nafion™ Polymer Permeability
Removed by Nafion™ Polymer
| Atmospheric Gases | H20 |
| Inorganics | NH3 |
| Organics | Alcohols, DMSO, THF |
Variable Losses
| Organics | Acids, Aldehydes, Amines, Ketones, Nitriles |
Nafion™ Polymer Im-Permeability
Totally Retained in Sample
| Atmospheric Gases | Ar, He, H2, N2, O2, O3 |
| Halogens | Br2, Cl2, F2, l2 |
| Hydrocarbons | Simple Forms (Alkanes) |
| Inorganic Acides | AHCL, HF, HNO3, H2SO4 |
| Other Organics | Aromatics, Esters, Ethers |
| Oxides | CO, CO2, SOX, NOX |
| Sulfur | COS, H2S, Mercaptans |
| Toxic Gases | COCL2, HCN, NOCl |
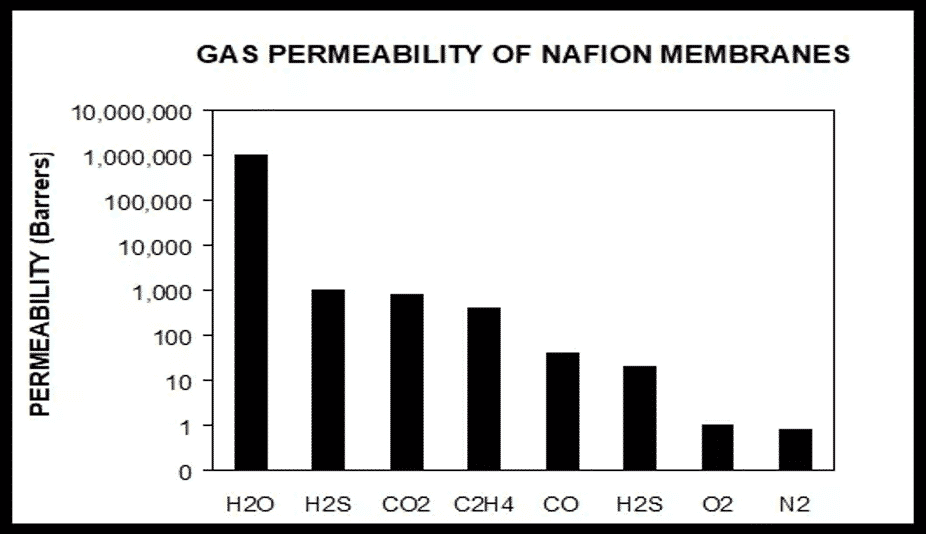
Gas Permeability of Nafion™ Polymer and Water
We are often asked about the relative selectivity of water in Nafion™ polymer relative to common gasses. Please see the graph for the relative gas permeability of water through Nafion versus some common gases. As you can see, Nafion is very effective at transporting only water while keeping the other gasses separated. For example, for H2S and CO2, water has a 1000 to 1 affinity and O2 and N2 a 1,000,000 to 1